HUMAN EXPERIMENTATION

The History of MKUltra

The Haunting History Of CIA Brainwashing Experiments: Project MKUltra
The events at Edgewood Arsenal involved a top-secret U.S. Army program during the Cold War that exposed thousands of soldiers to dangerous chemical and psychoactive agents to study their effects on human behavior and explore potential use in warfare. While elements of the program were coordinated with other agencies, including the CIA, it was primarily an Army operation.
Timeframe: The experiments were conducted from approximately 1948 to 1975 at the Edgewood Arsenal facility in Maryland.
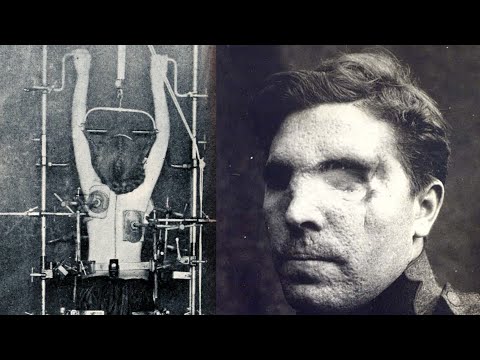
Scale: Around 7,000 U.S. military personnel and approximately 1,000 civilians were subjects in the tests, involving exposure to 254 different chemicals.
Agents Tested: A wide range of substances were used, including nerve agents like sarin, mustard gas, tear gas, and powerful hallucinogens such as LSD, PCP, and BZ.
Objective: The goal was to develop incapacitating agents that could be used to control or disable enemy forces without necessarily killing them, and to test protective equipment.
A major ethical breach was the lack of informed consent. Soldiers volunteered under the impression they were testing standard military gear or participating in harmless medical studies, often being told the drugs were like "aspirin". Once at the facility, some reported being pressured or threatened with court-martial if they refused to participate further.
The experiments had devastating and long-lasting effects on the participants.
Immediate Effects: Soldiers experienced temporary blindness, prolonged and terrifying hallucinations, paranoia, and sleeplessness. Films of the experiments showed men lapsing into delirium, unable to follow simple orders.
Long-Term Effects: Many veterans developed chronic psychological and physical issues, including neurological disorders, severe depression, insomnia, and suicidal thoughts.
Exposure and Accountability: The program ended in 1975 following congressional hearings that exposed the lack of informed consent and the program's failures. Decades later, many veterans struggled to get information about what they were exposed to or receive adequate compensation and medical support from the Department of Veterans Affairs (VA) for their related health problems.
Join us in advocating against the misuse of technology.
contact@targetedhumans.org
© 2025. All rights reserved.


Targeted Humans Inc.
