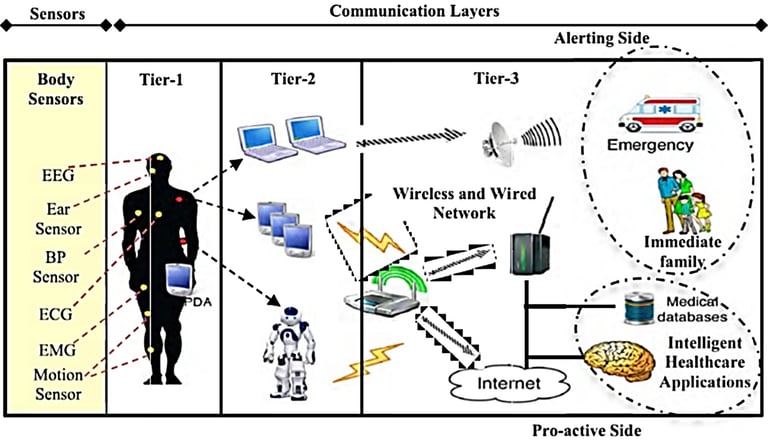
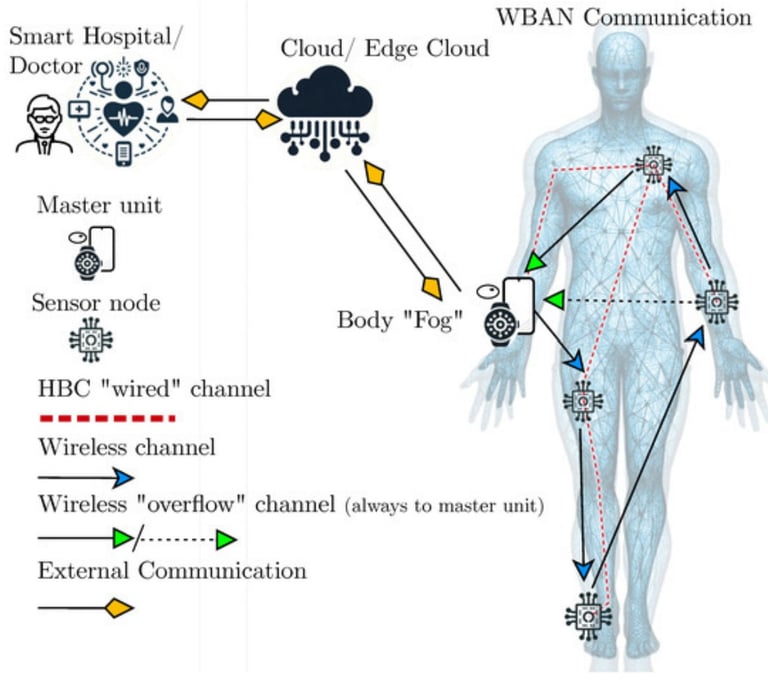
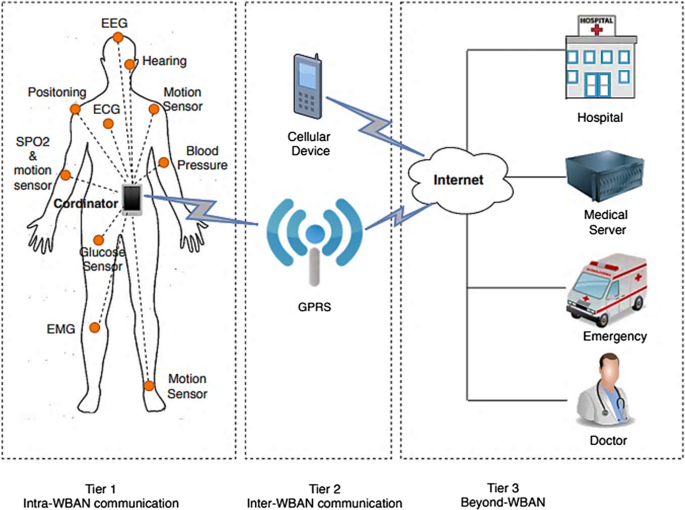
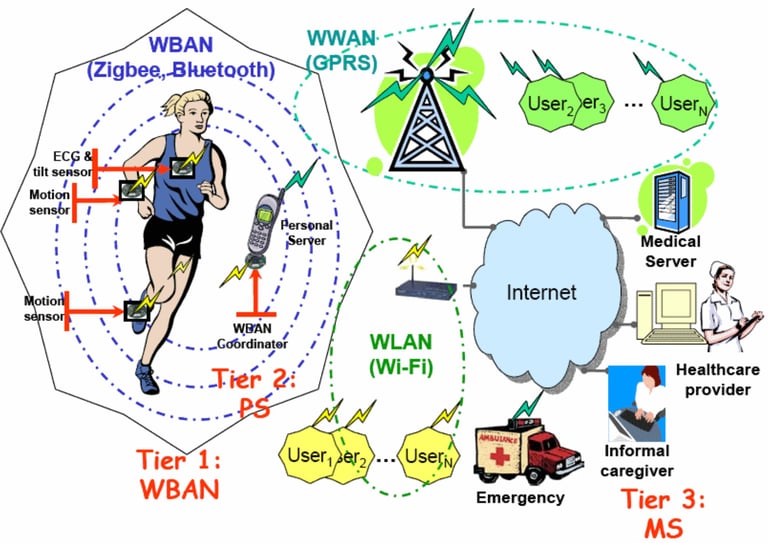
Connect2HealthFCC - Wireless Health and Medical Devices Background
The FCC's Office of Engineering and Technology (OET) conducts many activities relating to wireless medical devices, including equipment authorization (for products such as smartphones, car door remote controls, Wi-Fi devices, baby monitors, and personal computers); testing for radio frequency safety; and regulation of radio spectrum.
OET has a long history of working to enable health and medical devices. Some of its more recent accomplishments include:
MedRadio (Medical Device Radiocommunications Service): In the course of two rulemaking proceedings, the Commission allocated spectrum and adopted technical rules for innovative new body-worn and implanted medical radio devices that can provide a variety of diagnostic and therapeutic functions from diabetes and heart monitors to pacemakers and cardiofribrilators.
Medical Body Area Networks (MBANs): In 2012, the FCC released an Order to allocate spectrum for Medical Body Area Networks (MBANs), making the U.S. the first country in the world to make spectrum available for this specific usage. MBANs are networks of wireless sensors, often no bigger than a Band-Aid, which can transmit data on a patient's vital health indicators to their doctor or hospital. For more information, see the MBANs Fact Sheet and a listing of Frequency Bands for Medical Devices.
Medical Micropower Networks (MMNs): In 2011, the FCC adopted rules to enable a new generation of wireless medical devices that can be used to restore functions to paralyzed limbs. MMNs are ultra-low power wideband networks consisting of transmitters implanted in the body that take the place of damaged nerves, restoring sensation and mobility.
Retinal Implants: In November 2011, OET granted a waiver of the Commission's rules to Second Sight Medical Products, Inc. to allow it to obtain FCC certification for and market its Argus II Retinal Prosthesis System which is a medical implant system designed to treat profoundly blind people suffering from advanced retinal degenerative diseases. For more information, see the waiver (https://docs.fcc.gov/public/attachments/DA-11-1951A1.pdf)
Experimental Licensing Program: In May 2012, the FCC announced a plan to cut red tape and increase spectrum flexibility for testing new wireless health innovations, to speed new wireless health technologies to market. The new experimental licensing regime will create more flexibility and streamlined processes for testing new wireless medical devices.
FCC-FDA Memorandum of Understanding: In 2010, the FCC entered into an unprecedented partnership with the Food and Drug Administration, working together to ensure that communications-related medical innovations can swiftly and safely be brought to market. In June 2012, the FCC issued a letter in response to an inquiry from Reps. Walden, Bilbray, Blackburn, Burgess, Gingrey, and Pitts regarding wireless medical devices and the FCC's partnership with FDA, providing a comprehensive overview of activities that the two agencies have undertaken since enacting the MOU in 2010.
Frequency Bands for Medical Devices
Medical Radio Communications Service (MedRadio) Authorized under Part 95, subpart I
401-406 MHz Frequency Band – General Usage: medical devices for transmitting data containing operational, diagnostic and therapeutic information associated with a medical implant device or medical body worn devices
Frequency Bands for Specific Applications:
Medical Micropower Networks (MMNs): wireless medical devices that can be used to restore functions to paralyzed limbs
- 413-419 MHz
- 426-432 MHz
- 438-444 MHz
- 451-457 MHz
2360-2400 MHz Medical Body Area Networks (MBANs): networks of body-worn wireless sensors that transmit patient data to a health care provider
Please refer to the rules to determine specific technical and operational rules for each MedRadio frequency band. Wireless Medical Telemetry Service (WMTS): a short distance data communication service for transmitting patient medical information to a central monitoring location in a medical facility
Authorized under Part 95, subpart H
Frequency Bands:
- 608-614 MHz
- 1395-1400 MHz
- 1427-1429.5 MHz(location specific)
- 1429-1431.5 MHz (location specific)
Please refer to the rules to determine specific technical and operational rules for each MedRadio frequency band. Medical devices may also operate under the rules for unlicensed devices under Part 15 in any frequency band available under that Part.
IMPLANTS
Nano-Implants for Wireless Brain Interfacing
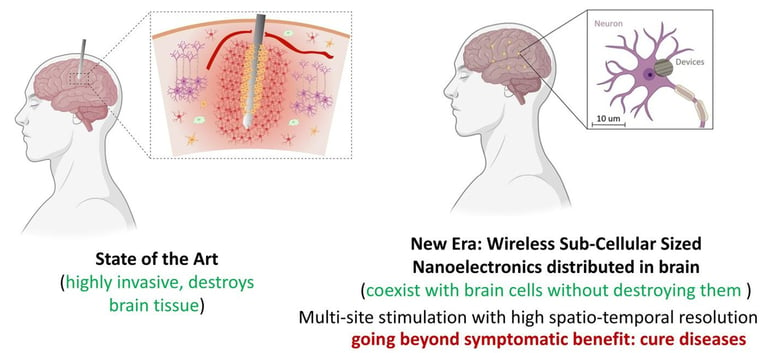
We are developing nano-devices using meta-materials that can non-invasively and remotely monitor and modulate our biological system. The requirements of the system are: 1> they should be as small as possible such that the volume displacement of tissue due to the placement of the device is minimal, 2> They should be untethered/wireless such that they can be remotely controlled. Such a device will sense the biological environment and send the information to a system outside the body in real time. The device will also have the capability to do internal analysis of the sensed data and depending on the analysis results, take further action such as electrical stimulation or drug delivery. The device will harvest energy from external applied fields for its functioning and also modulate the external fields for communicating sensed data.
The possibilities with such bioelectronic devices are endless, and we are exploring, among others, brain activity recording at a large scale with a precision of single neuron, activity recording in spinal cord and peripheral nervous system, monitoring tumor microenvironment, observing response to pathology development or external stimulus at a single cell level along with integrated functionalities such as stimulation and drug delivery.
https://www.media.mit.edu/projects/wireless-sensing/overview/
WHO IS ALLOWED TO OPERATE MEDICAL
IMPLANTS ACCORDING TO FCC REGULATIONS?
http://www.csgnetwork.com/micsfreqtable.html
Google Search: "enforcing FCC rules about who operates implant"
Medical Implant Communications Service Frequency Table
MICS Designated Frequency Ranges MICS = 402 to 405 MHz
This table is the frequency chart for the legal Medical Implant Communications Service. There is only 1 designated range (as of 4/1/2006 still) of frequencies. Each device must be registered for compliance and conflict avoidance. MICS, as it is called, uses certified private use grade, low power transmitters similar to Personal Radio Services equipment. However, the 402-405 MHz frequency band is available for MICS operations on a shared, secondary basis. The FCC determined that, compared to other available frequencies, the 402-405 MHz frequency band best meets the technical requirements of the MICS for a number of reasons. The 402-405 MHz frequencies have propagation characteristics conducive to the transmission of radio signals within the human body. In addition, equipment designed to operate in the 402-405 MHz band can fully satisfy the requirements of the MICS with respect to size, power, antenna performance, and receiver design. Further, the use of the 402-405 MHz band for the MICS is compatible with international frequency allocations. Finally, the use of the 402-405 MHz frequency band for the MICS does not pose a significant risk of interference to other radio operations in that band. MICS systems consist of the transmitters connected to medical implant devices, and programming, monitoring and control equipment. A Medical implant device is generally regarded as apparatus that is placed inside the human body for the purpose of performing diagnostic or therapeutic functions, such as cardiac pacemakers and defibrillators. Operation in the MICS is permitted by rule and without an individual license issued by the FCC. A person is permitted to operate medical implant transmitters connected to medical implant devices that have been implanted in that person by a duly authorized health care professional and medical implant programmer/control transmitters associated with their medical implant transmitter(s). Duly authorized health care professionals are permitted by rule to operate MICS transmitters. Manufacturers of medical implant devices and MICS transmitters and their representatives are authorized to operate transmitters in this service for the purpose of demonstrating such equipment to duly authorized health care professionals. No entity that is a foreign government or which is acting in its capacity as a representative of a foreign government is eligible to operate a MICS transmitter. The term 'duly authorized health care professional' means a physician or other individual authorized under state or federal law to provide health care services using medical implant devices. Operations that comply with the requirements of this part may be conducted under manual or automatic control.
The MICS was officially adopted by the FCC on October 10, 1999; this page was created the same day. The service rules for the equipment and use of the MICS include limitations on transmitter output power, out-of-band emissions, and protection of other services. Users of MICS transmitters must cooperate in the selection and use of channels in order to reduce interference and to make the most effective use of the authorized facilities. Most importantly, channels must be selected so as to avoid interference to other MICS transmissions. As a safeguard against such MICS to MICS interference, external medical implant programmer/control MICS transmitters must incorporate a mechanism for monitoring the channel or channels that the MICS system devices intend to occupy and, unless there is a medical implant event, may not initiate a MICS communications session unless certain 'access criteria' are met. A medical implant event is defined as an occurrence or the lack of an occurrence recognized by a medical implant device, or a duly authorized health care professional, that requires the transmission of data from a medical implant transmitter in order to protect the safety or well being of the person in whom the medical implant transmitter has been implanted. The Commission has clarified that regularly scheduled transmissions that are not instigated by a change in the patient’s medical condition do not qualify as medical implant events. A request for waiver to permit certification of implanted devices that emit periodic scheduled transmissions is pending. In addition, all MICS operations, as a consequence of their secondary status, must not cause harmful interference to stations in the Meteorological Aids, Meteorological Satellite, or Earth Exploration Satellite Services operating in the same or adjacent frequencies. Further, MICS stations must accept interference from such Meteorological Aids, Meteorological Satellite, or Earth Exploration Satellite Service stations. Any non-implanted MICS apparatus must be made available for inspection upon request by the FCC. Persons operating implanted medical implant transmitters must cooperate reasonably with the FCC in the resolution of interference complaints.
For complete licensing information, see the FCC license information, here. This is the link to the FCC personal radio services available. You may also find interest in the Family Radio Service (FRS) Frequency Table, General Mobile Radio Service (GMRS) Frequency Table, Multi-Use Service (MURS) Frequency Table, Wireless Medical Telemetry Service (WMTS) Frequency Table and the Citizens Band Radio (CB) Frequency Table. MICS is one of five Citizens Band Radio Services. The others are the (original) Citizens Band Radio Service at 27 MHz, the Wireless Medical Telemetry Service (WMTS) at 216-217 MHz, the Low Power Radio Service (LPRS) at 216-217 MHz, and the Family Radio Service (FRS) at 460 MHz.
FCC Personal Radio Services
Personal radio services provide short-range, low power radio for personal communications, radio signaling, and business communications not provided for in other wireless services. The range of applications is wide, spanning from varied one- and two way voice communications systems to non-voice data transmission devices used for monitoring patients or operating equipment by radio control. Licensing and eligibility rules vary. Some personal radio services require a license grant from the FCC, while others require only that you use equipment that is properly authorized under the FCC's rules. See specific service pages for the licensing and eligibility details about each individual service.
The personal radio services are:
218-219 MHz Service - One or two way communications for transmission of information to subscribers within a specific service area.
Citizens Band (CB) Radio Service - 1-5 mile range two-way voice communication for use in personal and business activities.
Family Radio Service (FRS) - 1 mile range Citizen Band service for family use in their neighborhood or during group outings
General Mobile Radio Service (GMRS) - 5-25 mile range Citizen Band service for family use in their neighborhood or during group outings.
Low Power Radio Service (LPRS) - private, one-way communications providing auditory assistance for persons with disability, language translation, and in educational settings, health care, law, and AMTS coast stations.
Maritime Survivor Locating Devices (MSLD) - used by people at risk of falling into the water to alert others of an emergency situation.
Medical Device Radiocommunication Service (MedRadio) - for transmitting data in support of diagnostic or therapeutic functions associated with implanted medical devices.
Multi-Use Radio Service (MURS) - private, two-way, short-distance voice or datacommunications service for personal or business activities of the general public.
Personal Locator Beacons (PLB) - used by hikers, and people in remote locations to alert search and rescue personnel of a distress situation.
Radio Control Radio Service (R/C) - one-way non-voice radio service for on/off operation of devices at places distant from the operator.
Wireless Medical Telemetry Service (WMTS) - for remote monitoring of patients' health through radio technology and transporting the data via a radio link to a remote location, such as a nurses' station.
"We are witnessing explosive growth in wireless medical technologies, including devices that control bodily functions and measure an array of physiological parameters. Even software, such as a smartphone application, can be considered a medical device depending on its function. Other types of wireless medical devices can communicate in some manner with landline networks, cellular systems, or broadband facilities that access the Internet."
-Fish and Richardson
https://www.fr.com/services/regulatory/mhealth-and-telemedicine/
Implant Technology
These rules are being violated every second of the day by the people who operate the Body Area Network connected to Targeted Individuals and by radio communications that operate the horrendous V2K assaults. There are no exceptions to these rules for any reason that can be read in these rules.
"A person is permitted to operate medical implant transmitters connected to medical implant devices that have been implanted in that person by a duly authorized health care professional and medical implant programmer/control transmitters associated with their medical implant transmitter(s). Duly authorized health care professionals are permitted by rule to operate MICS transmitters.",
NOTE: THIS DOES NOT SAY ORDINARY PERSONS WORKING IN MILITARY , LAW ENFORCEMENT, FIREMEN, VETERANS, DRUG ADDICTS, COPS, CAPS, CONTRACTORS or "VOLUNTEERS" or DISPATCHER ORGANIZED STALKERS OF ANY KIND CAN CONNECT OR OPERATE YOUR IMPLANTS. There was no legal authorization for the implants to be implanted.
YET, THIS HAPPENS EVERY HOUR OF EVERY DAY ANYONE IS IMPLANTED WITH THE BODY AREA NETWORK AND BECOMES A THING IN THE INTERNET OF THINGS.





This technology has not been released to the public as yet, it hasn't even been through clinical trials, that's what Targeted Individuals are being used for. Instead of Brown Shirted Hitler Youth, we have neighborhood mothers, fathers, kids, aunts, uncles, grandmothers and grandfathers using their phones to connect TI's when they are shopping and they are hit with microwave that operate their implants to make them fall down, have pain explode in their feet, or make their feet and legs go numb. They can make a TI lose control of their elimination. They can be hit the TI in the head with pain.
This has been going on for a at least 20 years now. Many TI's have been killed with this weaponization of the communication system. This is what Infragard and their partners do to people when they are out and about going to work, going to concerts or sporting events, or just going to the beach.










Some TI's have already been victim of this type of injection. This is a real experience in 2013. I made an appointment to go to the doctor. Like always, someone from the gov told them I was coming and the receptionist was told to connect me with her phone in some way. That became very obvious when the connection actually did connect.
The night before I went to the doctor, I received an injection in the middle of my chest in the night. There was a bump with redness all around.
I went to the doctor, checked in and sat down. The receptionist got her phone out and started using it. She went outside with her phone. I had to go to my car for something and when I re-entered the waiting room, my head went on fire. All around my skull, it was heated like it was on fire. It lasted about 10 seconds, but that was enough, I knew what she had done and I knew what the injection was for. The phone directed energy towards me that reacted with whatever the injection contained - metal particles. That had never ever happened to me before and it has never happened after that. However, I sill have part of the implant in my chest.
-Targeted Individual since 2004
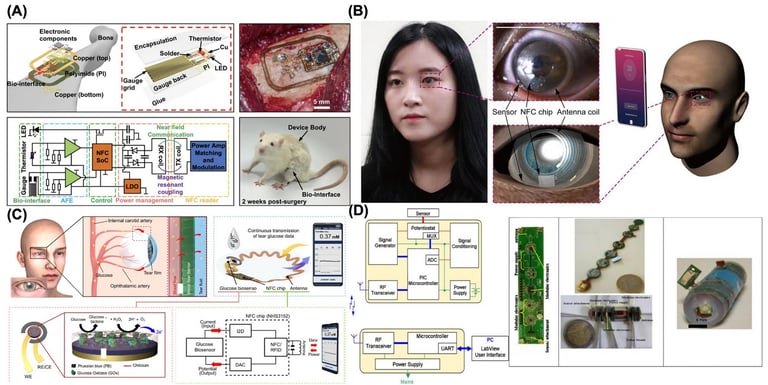


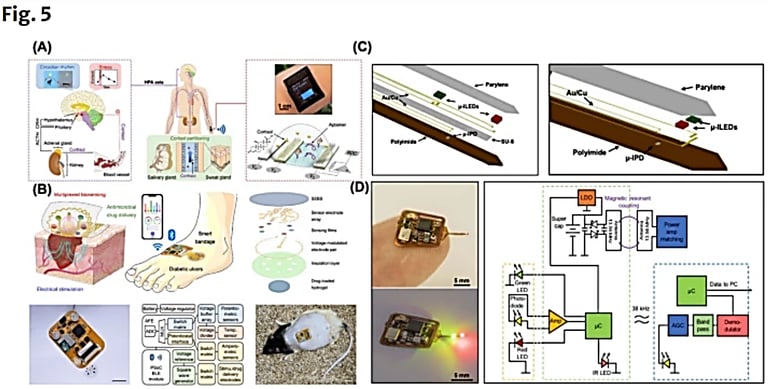
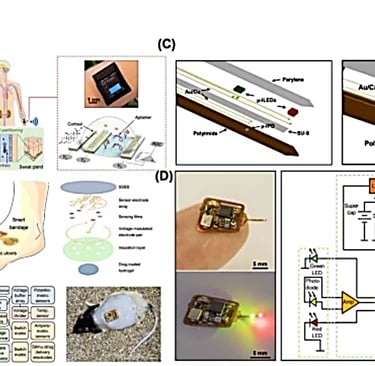
Technologies and applications in wireless biosensors for real-time health monitoring
https://link.springer.com/article/10.1007/s44258-024-00041-3#citeas
Published: 25 November 2024
Xu, Z., Hao, Y., Luo, A. et al. Technologies and applications in wireless biosensors for real-time health monitoring. Med-X 2, 24 (2024). https://doi.org/10.1007/s44258-024-00041-3
Implantable Sensors:
Wireless implantable, ingestible, or digestible biosensors allow for rigorous biophysical and biochemical monitoring, enabling real-time closed-loop interventions through in vivo measurements and continuous physiological detection [166]. Several biomedical applications exist for implantable sensors, ranging from neurological to gastroenterological pathways [167].

Technical and Feasibility Analysis
Power Discrepancy: WBAN energy harvesting devices are designed to generate minute amounts of power, typically in the milliwatt range, which is just enough to power low-energy medical sensors or recharge small internal batteries. In contrast, a single modern Bitcoin mining machine (ASIC) consumes thousands of watts (kilowatts) of power, an amount equivalent to several households.
Energy Sources: The energy sources used for WBANs are typically body movements (kinetic) or body heat (thermal), which are low-density and highly variable.
Computational Intensity: Cryptocurrency mining, particularly for Proof-of-Work systems like Bitcoin, relies on immense computational effort and the associated massive energy consumption to solve complex mathematical problems. The limited energy from WBANs cannot power the specialized, high-performance hardware needed for this task.
Related Concepts:
While direct energy harvesting for standard crypto mining is not practical, some related concepts have been explored in research and art projects:
Proof-of-Activity Patents: A Microsoft patent application from 2020 described a theoretical system where human body activity (like brain waves or body heat emitted when performing a task such as viewing an advertisement) could be used as "proof-of-work" to verify data and earn cryptocurrency, rather than using traditional intensive computation. This system uses the data of activity as proof, not the energy itself to power the computational process.
Art Projects: An art project by the Dutch "Institute of Human Obsolescence" (IoHO) in 2018 used special suits to capture the body heat from 37 people to power a small computer that mined a negligible amount of cryptocurrency over several hours. This was primarily a symbolic project to highlight the link between human labor and data production.
In summary, the power available from WBANs is fundamentally insufficient for the energy demands of cryptocurrency mining.
Performance Evaluation of Magnetic Resonance Coupling Method for Intra-Body Network (IBNet)
[I do not have the full document on this one.] THEORETICAL RESEEARCH!
Spurred by the advances in ultra-low-power electronics and communications, emerging implantable medical devices have led to new insights into long-term and continuous healthcare monitoring. For effective management of such medical devices, the intrabody network (IBNet) is becoming increasingly important. However, traditional means of intra-body communication (e.g., galvanic, capacitive, RF) are often affected by significant path loss due to tissue absorption, shadowing effect, environmental variations, instability of transmission quality, grounding issues, antenna size, etc. In pursuit of more suitable technology for the IBNet, the magnetic resonance (MR) coupling arose a great interest in the community since the magnetic permeability of the biological tissue is similar to that of the air, absence of an external reference or ground, and near-field operation.
In this paper, we present magnetic resonance (MR) coupling as a promising method for the intra-body network (IBNet). With seven healthy human subjects, we systematically compared MR coupling to traditional intra-body communication methods (galvanic, capacitive, and RF). The study revealed that MR coupling could effectively send or receive signals (power/data) in biological tissue, with a maximum path loss (PL) less than 33 dB (i.e., at 13.56 MHz). Such path loss was lower than galvanic, capacitive, or RF couplings for the same distance. The angular orientation between the transmitter and the receiver coils showed minor variation in the path loss (0.19 ≤ ∆PL ≤ 0.62 dB) but observed some dependency on the distance (0.05 dB/cm). Different postures during the MR coupling essentially did not affect performance (∆PL ≤ 0.21 dB). The multi-nodal transmission between a single transmitter and multiple receivers showed that the signal could be simultaneously delivered, demonstrating a potential for communication, sensing, and powering wearable and implantable devices. Overall, the MR coupling conserves energy for long-term implants by enabling low-power communication at lower path loss in the human body. https://www.embs.org/featured-articles/performance-evaluation-of-magnetic-resonance-coupling-method-for-intra-body-network-ibnet/
Magnetic Resonance Coupling for Intra Body Network (1BNet)
Mining your body's energy for Crypto Currency? [THEORETICAL]

Join us in advocating against the misuse of technology.
contact@targetedhumans.org
© 2025. All rights reserved.


Targeted Humans Inc.